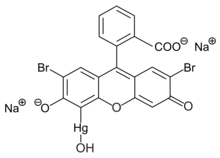
Merbromin
| |

| |
| |
| Names | |
|---|---|
| IUPAC name
dibromohydroxymercurifluorescein
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.486 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H8Br2HgNa2O6 | |
| Molar mass | 750.658 g·mol−1 |
| Appearance | dark red liquid |
| Pharmacology | |
| D08AK04 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, dangerous for the environment |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P264, P273, P280, P284, P301+P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Merbromin (marketed as Mercurochrome, Merbromine, Mercurocol, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept and Cinfacromin) is an organomercuric disodium salt compound used as a topical antiseptic for minor cuts and scrapes and as a biological dye. Readily available in most countries, it is no longer sold in Switzerland, Brazil, France, Iran, Germany, Denmark, or the United States, due to its mercury content.[1][2]
Uses
[edit]Merbromin's best-known use is as a topical antiseptic to treat minor wounds, burns, and scratches.[3] It is also used in the antisepsis of the umbilical cord,[4] and the antisepsis of wounds with inhibited scar formation, such as neuropathic ulcers and diabetic foot sores.[5] When applied on a wound, it stains the skin a distinctive carmine red, which can persist through repeated washings. Due to its persistence and to its lethality to bacteria, Merbromin is useful on infections of the fingernail or toenail.
In 1998, the U.S. Food and Drug Administration reclassified merbromin from "generally recognized as safe" to "untested," due to a lack of recent studies or updated supporting information.[6] Consequently, its use in the United States has been superseded by other agents (e.g., povidone iodine, benzalkonium chloride, chloroxylenol).
Synthesis
[edit]Merbromin is synthesized by combining dibromofluorescein with mercuric acetate and sodium hydroxide or, alternatively, through action of the mercuric acetate upon (or combining with) sodium dibromofluorescein. Because of its anionic character, it is chemically incompatible with acids, the majority of alkaloid salts and most local anesthetics.[7]
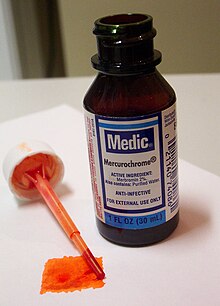
Mercurochrome
[edit]Merbromin is sold under the trade name Mercurochrome (in which the suffix "-chrome" denotes "color"[8]). The name is also commonly used for over-the-counter antiseptic solutions consisting of merbromin (typically at 2% concentration) dissolved in either ethyl alcohol (tincture) or water (aqueous).
Its antiseptic qualities were discovered in 1918 by Hugh H. Young, a physician at Johns Hopkins Hospital.[9] The chemical soon became popular among parents and physicians for everyday antiseptic uses, in part because the dye component made it easy to see where the antiseptic had been applied.
On 19 October 1998, citing potential for mercury poisoning, the US Food and Drug Administration (FDA) reclassified merbromin from "generally recognized as safe" to "untested," effectively halting its distribution within the United States.[1] Sales were subsequently halted in Brazil (2001), Germany (2003),[2] and France (2006).[citation needed] It remains readily available in most other countries.[citation needed]
Within the United States, products such as Humco Mercuroclear ("Aqueous solution of benzalkonium chloride and lidocaine hydrochloride") play on the brand recognition history of Mercurochrome but substitute other ingredients with similar properties.[10] In Canada, Jean Coutu Group markets a chlorhexidine solution under the name Mercurochrome.[11]
See also
[edit]- Nitromersol, an organomercury antiseptic and antifungal agent
- Phenyl mercuric nitrate
- Thiomersal, also known as Thimerosal or Merthiolate
References
[edit]- ^ a b "Quantitative and Qualitative Analysis of Mercury Compounds in the List". Federal Food, Drug, and Cosmetic Act (FD&C Act). U.S. Food and Drug Administration. 30 April 2009.
- ^ a b "Merbromin im Spiegel der Expertenmeinungen". [idiomatically: Merbromin in Light of Expert Opinion]. 22 September 2003.
- ^ "Prospecto autorizado Mercromina Film ®" (PDF). Retrieved 15 May 2013.[permanent dead link]
- ^ Sellares Casas, E; et al. "Eficacia de una aplicación frente a 3 de merbromina en el tiempo de caída del cordón". Acta Pediátrica Española. 60 (9).
- ^ Gaitan Enríquez, J (September 1997). "Merbromina como tratamiento de elección en úlceras de pie diabético". Clínica Rural. 497.
Se ha estudiado en 72 pacientes la eficacia de la merbromina comparativamente con la clorhexidina como antisépticos usados en la curación de múltiples afecciones en el pie diabético. Con el uso de merbromina se consigue disminuir apreciablemente el tiempo de cicatrización de las heridas, y se ha observado también una ausencia de complicaciones en los casos estudiados.
- ^ "Status of Certain Additional Over-the-Counter Drug Category II and III Active Ingredients". Federal Register. 63 (77). 22 April 1998.
- ^ Budavary, Susan, ed. (1989). The Merck Index, An Encyclopedia of Chemicals, Drugs, and Biologicals (11 ed.). Rahway, New Jersey, USA.: Merck & Co. ISBN 091191028X.
- ^ "Mercurochrome". Trésor de la Langue Française informatisé (in French). Centre National de Ressources Textuelles et Lexicales. Retrieved 11 April 2020.
- ^ Young, Hugh (15 November 1919). "A New Germicide for Use in the Genito-Urinary Tract: Mercurochrome-220". Journal of the American Medical Association. 73 (20): 1483–1491. doi:10.1001/jama.1919.02610460001001. Retrieved 8 July 2022.
- ^ "Mercuroclear MSDS" (PDF). Humco. Archived from the original (PDF) on 3 March 2016.
- ^ "Antiseptique solution de premiers soins sans mercure, 25 ml – Personnelle : Désinfectant". Archived from the original on 2 April 2019. Retrieved 2 April 2019.

